- For US Healthcare Professionals Only
- Important Safety Information
- Full Prescribing Information
- INVEGA HAFYERA™ Consumer Site
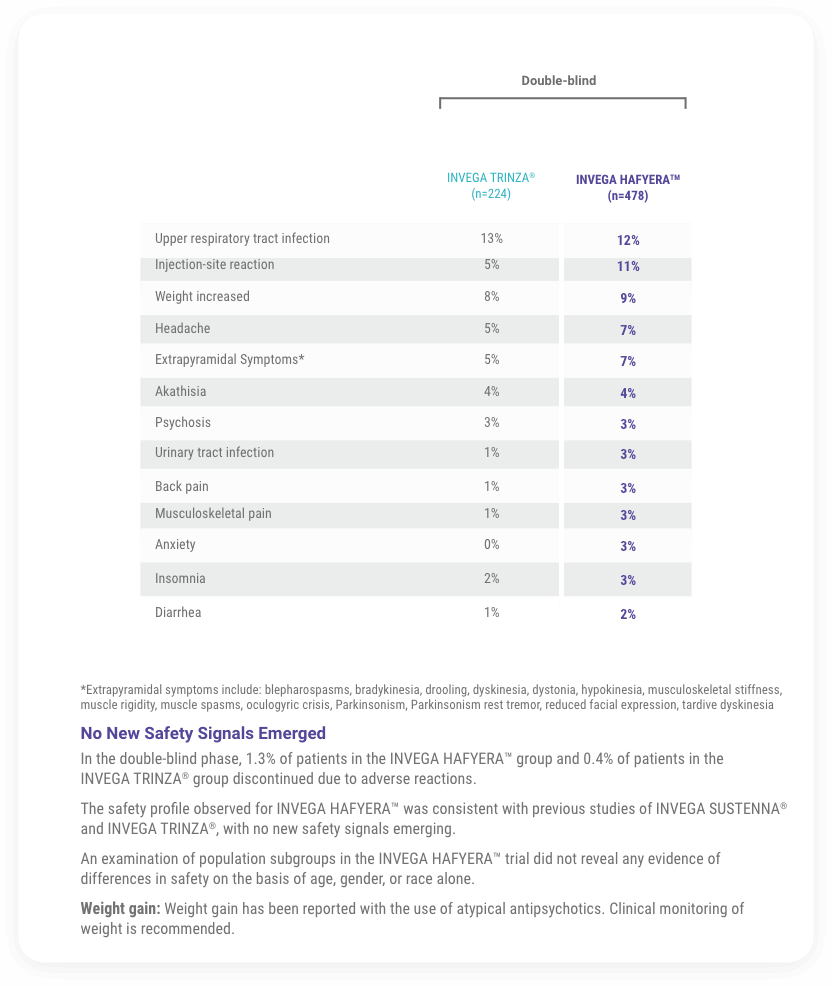
The Safety Profile of INVEGA HAFYERA™ Was Similar to That Seen With INVEGA TRINZA® (paliperidone palmitate) in the Double-blind Phase of the Study
Treatment-emergent Adverse Reactions (≥2%) During Noninferiority Study1
Patient Ratings of Injection-site Pain Were Mild
Patient Ratings of Injection-site Pain Were Mild, According to Visual Analog Scale1

Investigator ratings of injection site:
Induration, redness and swelling were observed in 13% in the INVEGA HAFYERA™ group and 9% in the INVEGA TRINZA® group during the double-blind phase.1
REFERENCES: 1. INVEGA HAFYERA™ [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; August 2021. 2. Data on file. CSR. Janssen Pharmaceuticals, Inc., Titusville, NJ. 2020.