- For US Healthcare Professionals Only
- Important Safety Information
- Full Prescribing Information
- INVEGA HAFYERA™ Consumer Site
Incidence of Extrapyramidal Symptoms Was Similar Between INVEGA HAFYERA™ and INVEGA TRINZA® (paliperidone palmitate)
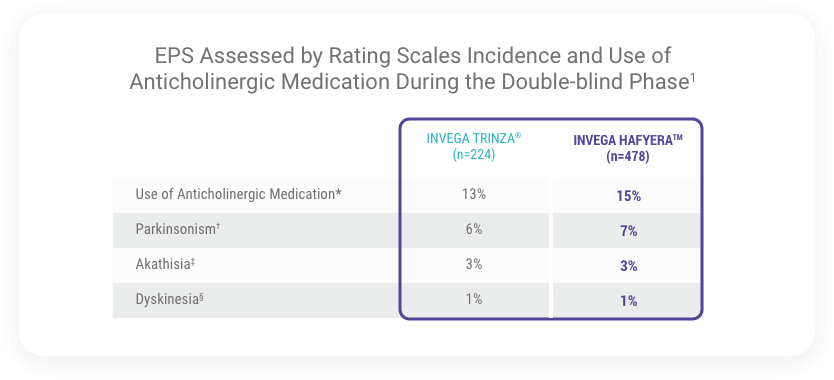
*Use of anti-EPS medication during the double-blind phase.
†Percentage of patients with Simpson-Angus Scale Global Score >0.3 (Global Score defined as the total sum of items score divided by the number of items).
‡Percentage of patients with Barnes Akathisia Rating Scale Global Clinical Rating Score ≥2.
§Percentage of patients with a score ≥3 on any of the first 7 items or a score ≥2 on 2 or more of any of the first 7 items of the Abnormal Involuntary Movement Scale.
Note: Percentages are calculated based on the number of patients in the DB Safety analysis set per treatment group.
EPS=extrapyramidal symptoms.
DB=double-blind phase.
REFERENCE: 1. INVEGA HAFYERA™ [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; August 2021.