- For US Healthcare Professionals Only
- Important Safety Information
- Full Prescribing Information
- INVEGA HAFYERA™ Consumer Site
Absorption & Distribution
- Due to its extremely low water solubility, INVEGA HAFYERA™ dissolves slowly after injection before being hydrolyzed and absorbed into systemic circulation. The release profile and dosing regimen of INVEGA HAFYERA™ result in sustained therapeutic concentrations over 6 months1
Uniquely Designed Long-Acting Formulations
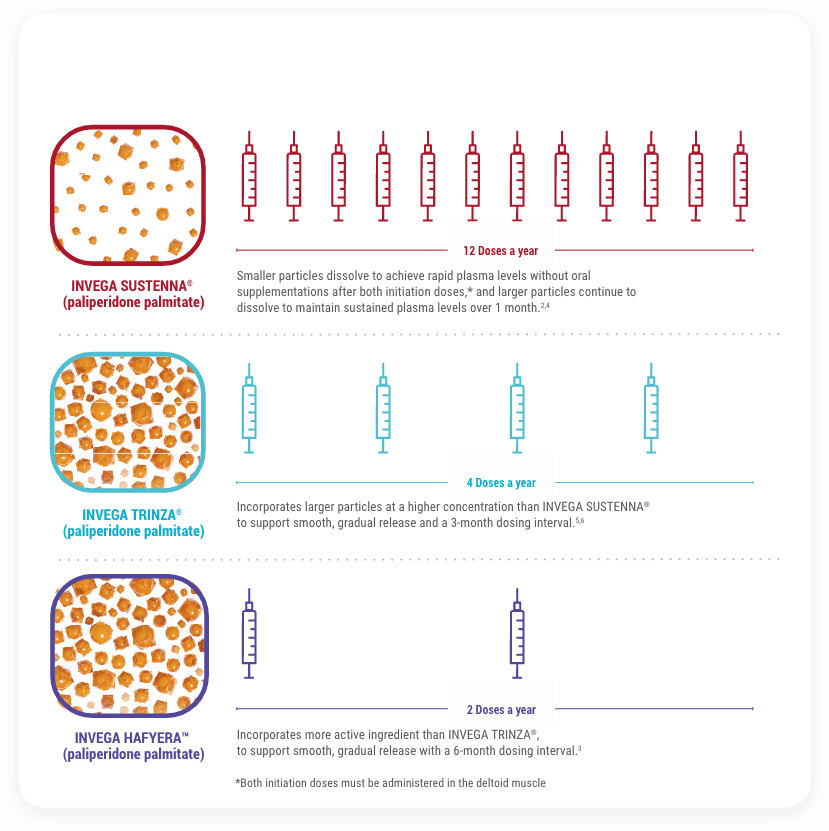
With INVEGA HAFYERA™, You Can Be Confident Patients Receive 6 Months of Medication
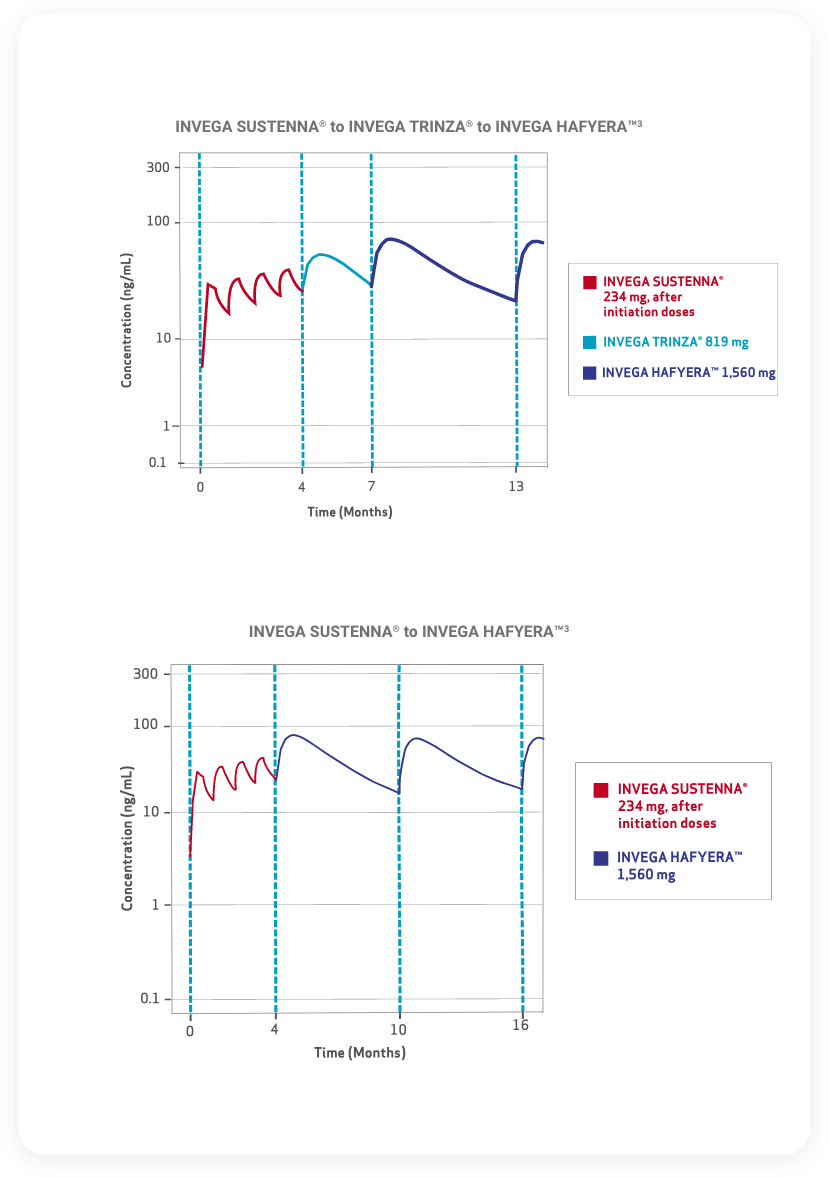
- Plasma concentrations above represent deltoid injections for INVEGA SUSTENNA® and INVEGA TRINZA®. INVEGA HAFYERA™ must be injected into the gluteal muscle only; do not administer by any other route
- Due to the difference in median pharmacokinetic profiles between INVEGA SUSTENNA®, INVEGA TRINZA®, and INVEGA HAFYERA™, caution should be exercised when making a direct comparison of their pharmacokinetic properties.1,2,5
- Correlation to clinical effect has not been established
REFERENCES: 1. INVEGA HAFYERA™ [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; August 2021. 2. INVEGA SUSTENNA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2021. 3. Data on file. Janssen Pharmaceuticals, Inc., Titusville, NJ. 4. Bishara D, Taylor D. Paliperidone palmitate. Br J Clin Pharmacol. 2011;3:75-78. 5. INVEGA TRINZA® [Prescribing Information]. Titusville, NJ: Pharmaceuticals, Inc.; February 2021. 6. Ravenstijn P, Remmerie B, Savitz A, et al. Pharmacokinetics, safety, and tolerability of paliperidone palmitate 3-month formulation in patients with schizophrenia: A phase-1, single-dose, randomized, open-label study. J Clin Pharmacol. 2016;56(3):330-339.