- For US Healthcare Professionals Only
- Important Safety Information
- Full Prescribing Information
- INVEGA HAFYERA™ Consumer Site
INVEGA HAFYERA® Was Evaluated in a 1-year, Noninferiority Study and a 2-year, Open-label Extension Study
In a Noninferiority Study, INVEGA HAFYERA® Delayed Relapse as Effectively as INVEGA TRINZA®
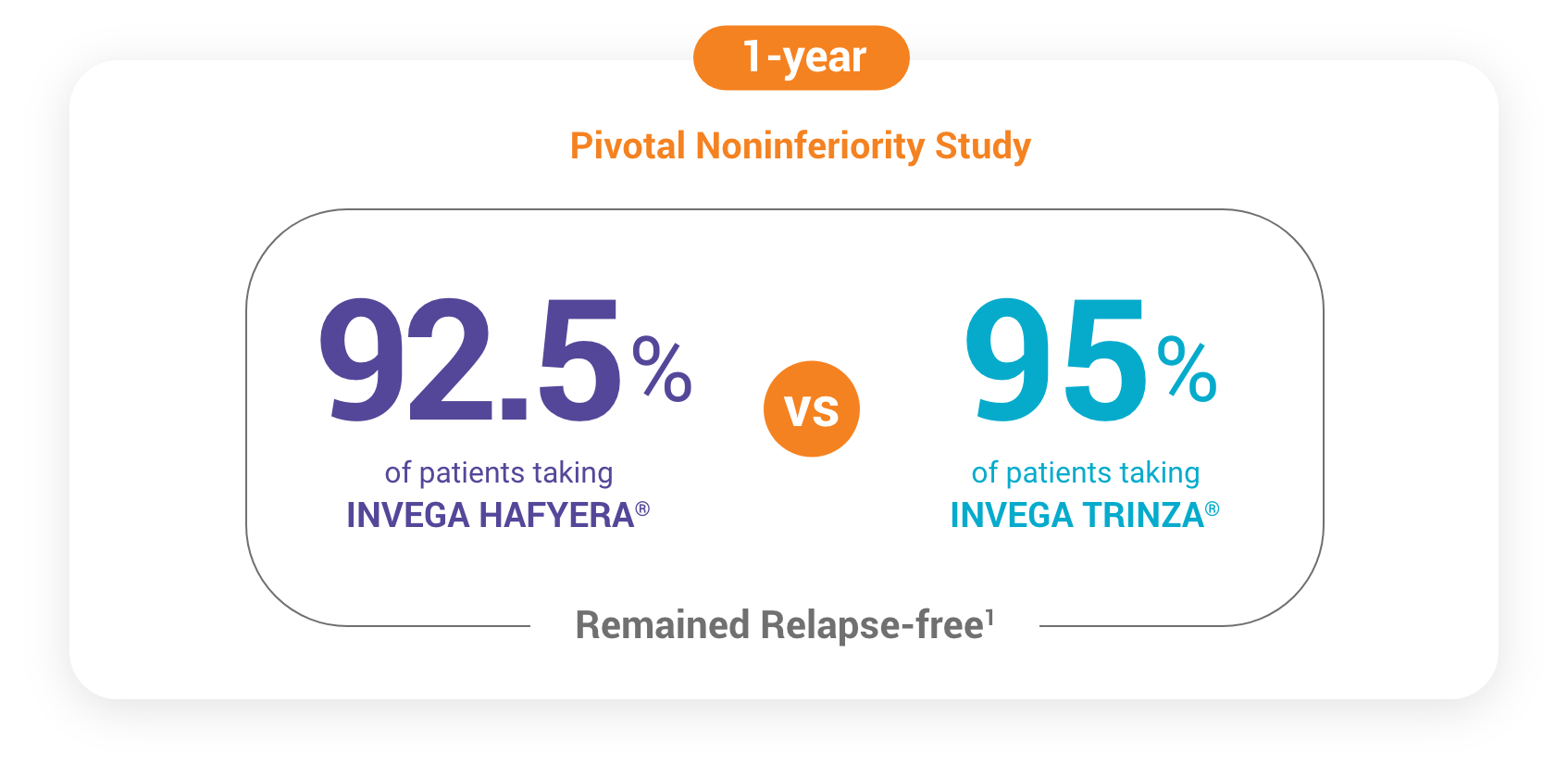
Results of a 12-month, randomized, double-blind, noninferiority trial. The primary efficacy variable was time to first relapse in the double-blind study.
A total of 702 stabilized patients were randomized in a 2:1 ratio to receive INVEGA HAFYERA® (n=478) or INVEGA TRINZA® (n=224) over the 12-month, double-blind study.2
Safety and Adverse Reactions: Noninferiority Study
- In the double-blind phase, 1.3% of patients in the INVEGA HAFYERA® group and 0.4% of patients in the INVEGA TRINZA® group discontinued due to adverse events
- The most common adverse reactions (≥5%) in the INVEGA HAFYERA® group were upper respiratory tract infection (12%), injection-site reaction (11%), weight increased (9%), headache (7%), and extrapyramidal symptoms† (7%)
- The most common adverse reactions (≥5%) in the INVEGA TRINZA® group were upper respiratory tract infection (13%), weight increased (8%), injection-site reaction (5%), extrapyramidal symptoms* (5%), and headache (5%)
*Extrapyramidal symptoms include: blepharospasms, bradykinesia, drooling, dyskinesia, dystonia, hypokinesia, musculoskeletal stiffness, muscle rigidity, muscle spasms, oculogyric crisis, parkinsonism, parkinsonism rest tremor, reduced facial expression, tardive dyskinesia.

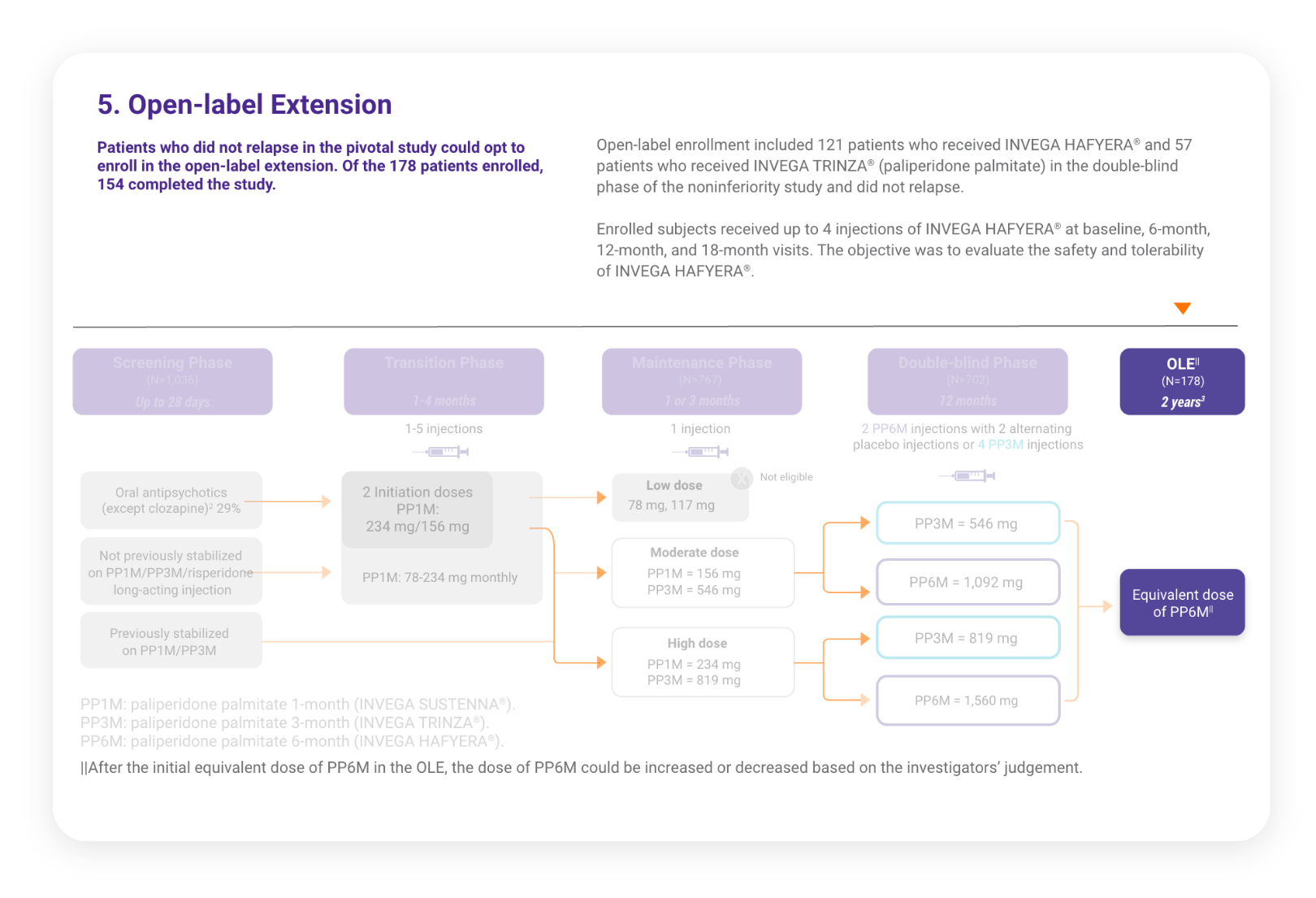
Patients Who Were Relapse-free for 1 Year in the Pivotal Study Were Eligible to Enter the Open-label Extension Study for 2 Years3
178 patients who were relapse-free on INVEGA HAFYERA® (n=121) or INVEGA TRINZA® (n=57) in the double-blind phase chose to continue treatment with INVEGA HAFYERA® in the open-label extension.3
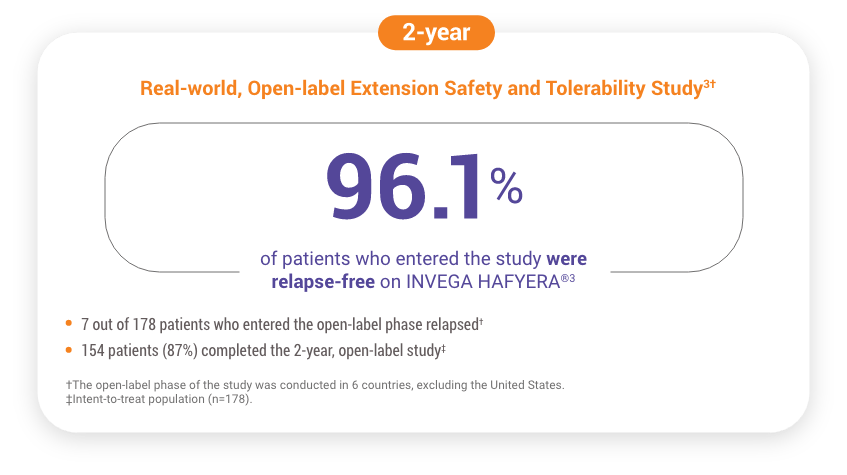
Safety and Adverse Events: Open-label Extension Study
- The most common (≥5%) treatment-emergent adverse events were headache (13.5%), blood prolactin increased (10.7%), hyperprolactinemia (7.3%), diarrhea (6.2%), weight increased (5.1%), and nasopharyngitis (5.1%)3§
§In the OLE design, investigators were not blinded to prolactin laboratory results. Comparisons between double-blind studies and OLE studies should
What might you expect for an adult patient with schizophrenia over the course of 3 years?
Patrick, a person living with schizophrenia
Patients Who Completed the Open-label Extension Study Maintained Treatment for a Total of 3 Years1-3
Mean PANSS Total Scores
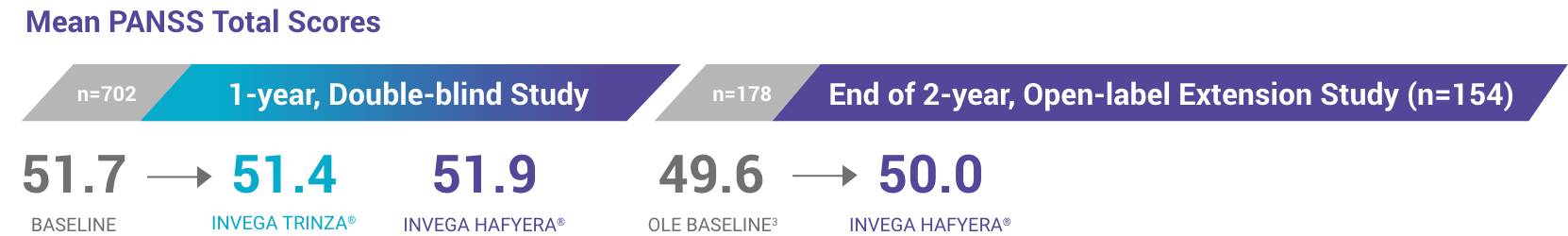
178 patients who were relapse-free for 1 year on INVEGA HAFYERA™ (n=121) or INVEGA TRINZA® (n=57) in the double-blind study chose to continue treatment with INVEGA HAFYERA™ in the open-label extension.
At study entry, all patients were required to have a PANSS total score ≤70.2
Study Design
Click the button below for a walk-through of each phase of the noninferiority and OLE studies.
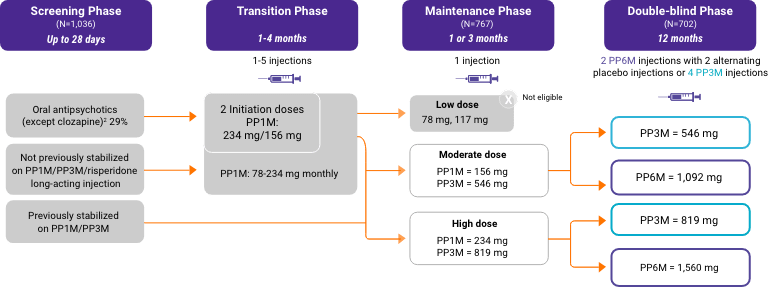

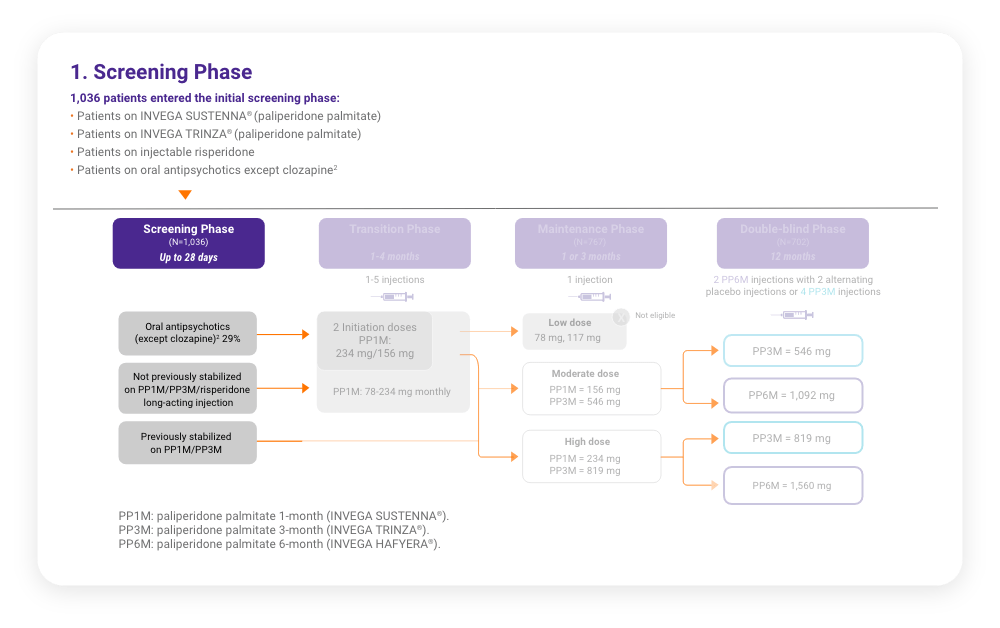
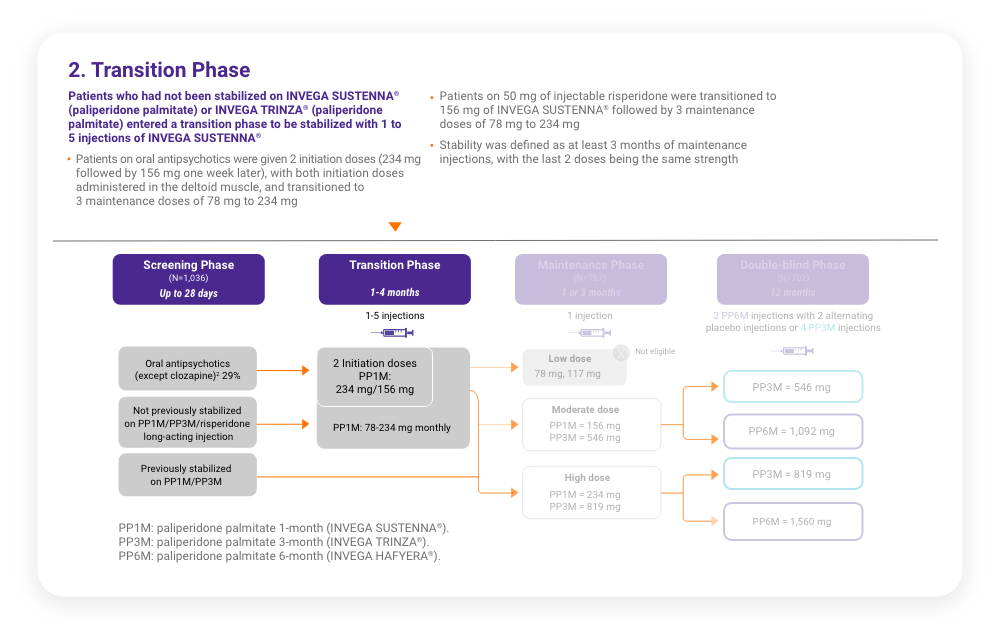
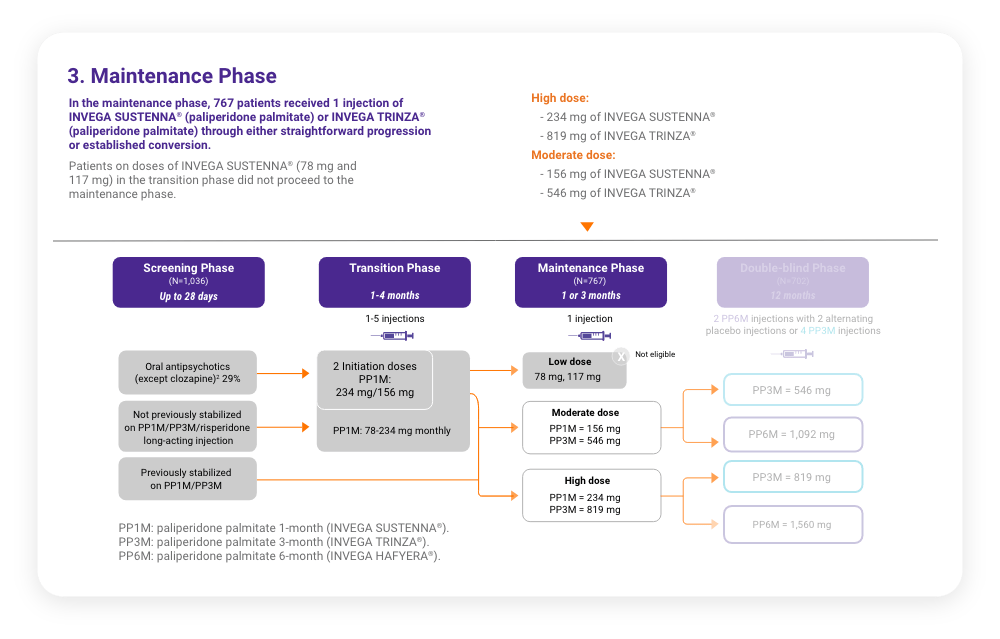
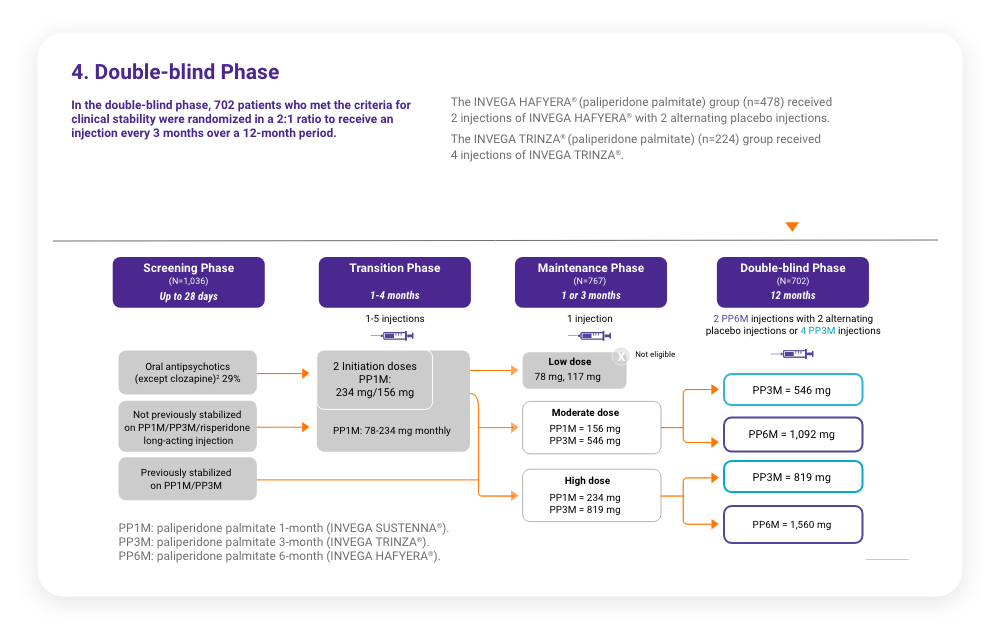

A total of 702 stabilized patients were randomized in a 2:1 ratio to receive INVEGA HAFYERA® (n=478) or INVEGA TRINZA® (n=224) over the 12-month, double-blind study.
Study Criteria
Pivotal Study Key Inclusion Criteria1,2
- Age: 18 to 70 years
- Diagnosed with schizophrenia (per DSM-5) for at least 6 months before screening
- Receiving treatment with INVEGA SUSTENNA®, INVEGA TRINZA®, injectable risperidone, or any oral antipsychotic
- Total PANSS scores of <70 points at screening and at randomization
Open-label Extension Key Inclusion Criteria3
- Completed the double-blind phase of pivotal study without relapse and willing to continue treatment with INVEGA HAFYERA™
Key Exclusion Criteria2
- Receiving any form of involuntary treatment
- Suicide attempt within 12 months before screening or imminent risk of suicide or violent behavior
- DSM-5 diagnosis of moderate or severe substance use disorder (except for nicotine and caffeine) within 6 months of screening
- History of NMS or TD
- Unstable medical conditions
- History of unresponsiveness or intolerance to paliperidone/risperidone
Relapse Criteria1-3
Relapse was defined as any of the following:
- Psychiatric hospitalization
- Increase of ≥25% in total PANSS score from randomization for 2 consecutive assessments (if baseline score was >40) (pivotal study only)
- 10-point increase in total PANSS score for 2 consecutive assessments (if baseline score was ≤40) (pivotal study only)
- Deliberate self-injury, violent behavior, or suicidal/homicidal ideation
- Score of ≥5 (if the maximum baseline score was ≤3) or ≥6 (if the maximum baseline score was 4) on 2 consecutive assessments of the specific PANSS items
- Emergency department/room/ward visit due to a worsening of the subject’s symptoms of schizophrenia (OLE only)
DSM-5=Diagnostic and Statistical Manual of Mental Disorders, 5th edition
NMS=neuroleptic malignant syndrome
OLE=open-label extension
PANSS=Positive and Negative Syndrome Scale
REFERENCES: 1. INVEGA HAFYERA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; August 2021. 2. Najarian D, Sanga P, Wang S, et al. A randomized, double-blind, multicenter, noninferiority study comparing paliperidone palmitate 6-month versus the 3-month long-acting injectable in patients with schizophrenia. Int J Neuropsychopharmacol. 2022;25(3):238-251. 3. Data on file. Janssen Pharmaceuticals, Inc., Titusville, NJ.