- For US Healthcare Professionals Only
- Important Safety Information
- Full Prescribing Information
- INVEGA HAFYERA™ Consumer Site
Changes in Fasting Glucose Levels Were Similar Between INVEGA HAFYERA™ and INVEGA TRINZA®
Change in Fasting Glucose From the Randomized, Double-blind Phase1
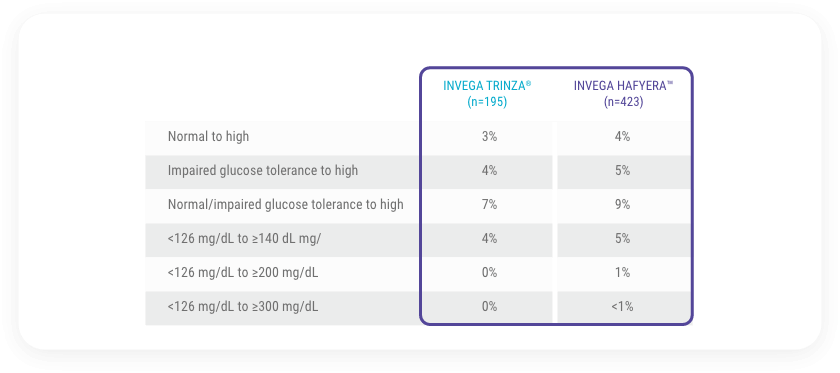
Table reflects subjects with paired fasting data (baseline and any post-baseline assessment).
Using the conversion factor (1 mg/dL=0.05551 mmol/L), the ADA-specified limits are as follows:
Normal: <100 mg/dL (<5.551 mmol/L)
Impaired: ≥100 mg/dL (≥5.551 mmol/L) to <126 mg/dL (<6.994 mmol/L)
High: ≥126 mg/dL (≥6.994 mmol/L)
126 mg/dL=6.994 mmol/L; 140 mg/dL=7.771 mmol/L; 200 mg/dL=11.102 mmol/L; 300 mg/dL=16.653 mmol/L
ADA=American Diabetes Association.
Hyperglycemia and diabetes mellitus
- Monitor for symptoms of hyperglycemia, including polydipsia, polyuria, polyphagia, and weakness
- Monitor glucose regularly in patients with diabetes or at risk for diabetes
Changes in Fasting Lipids Were Similar Between INVEGA HAFYERA™ and INVEGA TRINZA®
Shifts in Fasting Lipids in the Double-blind Phase From the Randomized, Active-controlled Study1

For each fasting parameter, patients with both baseline (DB) record and any post-baseline (DB) record during the double-blind phase are included in the denominator.
DB=double-blind phase.
Dyslipidemia
- Undesirable alterations in lipids have been observed in patients treated with atypical antipsychotics.
REFERENCE: 1. INVEGA HAFYERA™ [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; August 2021.